From: COVID-19
On Jan. 13, 2022, the U.S. Supreme Court dissolved the temporary injunctions blocking enforcement of the Centers for Medicare & Medicaid Services (CMS) emergency rule requiring COVID-19 vaccination of certain health care workers. As a result, the emergency rule was reinstated and can now be enforced as written.
CMS issued the following three pieces of guidance related to the emergency rule that apply to different groups of states. This guidance sets forth timelines and standards for compliance with the CMS emergency rule.
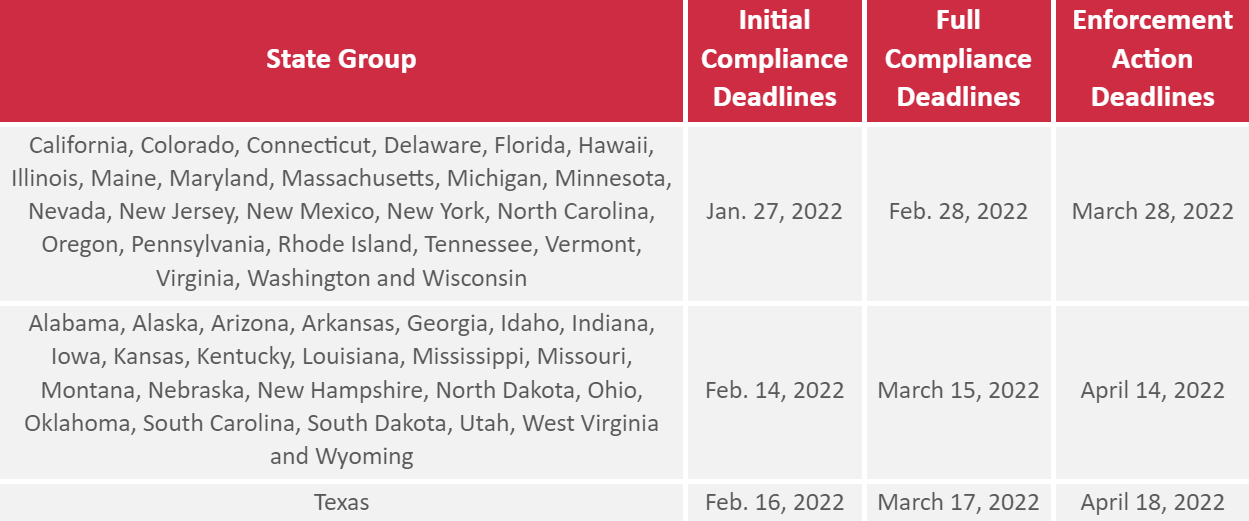
- QSO-22-07-ALL applies to California, Colorado, Connecticut, Delaware, Florida, Hawaii, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Jersey, New Mexico, New York, North Carolina, Oregon, Pennsylvania, Rhode Island, Tennessee, Vermont, Virginia, Washington and Wisconsin.
- QSO-22-09-ALL applies to Alabama, Alaska, Arizona, Arkansas, Georgia, Idaho, Indiana, Iowa, Kansas, Kentucky, Louisiana, Mississippi, Missouri, Montana, Nebraska, New Hampshire, North Dakota, Ohio, Oklahoma, South Carolina, South Dakota, Utah, West Virginia and Wyoming.
- QSO-22-11-ALL applies to Texas.
Action Steps
The emergency rule’s planned timeline has been adjusted due to the litigation. The specific compliance deadlines that apply vary within each state group. Facilities may be subject to enforcement action for failure to maintain 100% compliance with the CMS emergency rule by the applicable deadlines. Termination of participation from the Medicare and Medicaid programs is possible, although CMS has stated that noncompliant facilities will be given the opportunity to become compliant.
The CMS rule applies to Medicare- and Medicaid-certified provider and supplier types that are regulated under the Medicare health and safety standards, including hospitals, clinics and long-term care facilities. These facilities must establish a policy ensuring that all eligible staff is vaccinated against COVID-19 and ensure that staff has obtained the necessary doses to be fully vaccinated against COVID-19 within certain time frames.
The regulation provides for exemptions based on recognized medical conditions or religious beliefs. However, there is no weekly testing exception for unvaccinated workers.
Adjusted Enforcement Timeline
Under the rule’s planned timeline, staff members had to receive the first dose of a two-dose COVID-19 vaccine or a one-dose COVID-19 vaccine prior to providing any care, treatment or other services by Dec. 6, 2021, and the necessary shots to be fully vaccinated—either two doses of Pfizer-BioNTech or Moderna or one dose of Johnson & Johnson—by Jan. 4, 2022. However, this planned timeline has been adjusted due to the litigation.
The initial and full compliance deadlines that apply vary within each state group, as follows:
Initial Compliance
By the applicable initial compliance deadline, health care facilities must demonstrate that:
- A COVID-19 vaccination policy has been developed and implemented; and
- 100% of staff have received at least one dose of a COVID-19 vaccine, a pending request for (or been granted) a qualifying exemption or a temporary delay as recommended by the Centers for Disease Control and Prevention (CDC).
A facility that is above 80% and has a plan to achieve a 100% staff vaccination rate within 60 days would not be subject to additional enforcement action.
Full Compliance
By the applicable full compliance deadline, health care facilities must demonstrate that 100% of staff have completed the COVID-19 vaccine series, been granted a qualifying exemption or received a temporary delay as recommended by the CDC. A facility that is above 90% and has a plan to achieve a 100% staff vaccination rate within 30 days would not be subject to additional enforcement action.
Enforcement Action
Facilities may be subject to enforcement action for failure to maintain 100% compliance with the CMS emergency rule after the applicable enforcement action deadline. Termination of participation from the Medicare and Medicaid programs is possible, although CMS has stated that noncompliant facilities will be given the opportunity to become compliant.
Highlights
By the applicable initial compliance deadline, health care facilities must have implemented a COVID-19 vaccination policy and ensure that staff has received at least one dose of a COVID-19 vaccine.
By the applicable full compliance deadline, health care facilities must ensure that all staff have completed the COVID-19 vaccine series.
Enforcement action may apply if 100% of staff is not compliant by required deadlines.
Important Dates
January 13, 2022
The U.S. Supreme Court dissolved temporary federal court injunctions blocking enforcement of the emergency rule.
April 18, 2022
Enforcement action may apply if facilities are not fully compliant by this deadline.